Sodium Hydroxide Molar Mass
Properties of Sodium Hydroxide NaOH In order to describe its uses it is necessary to describe the features that caustic soda is used in a variety of industries due to these chemical and physical features. When dissolved in water or neutralized with acid it releases a significant amount of heat which could ignite combustible objects.
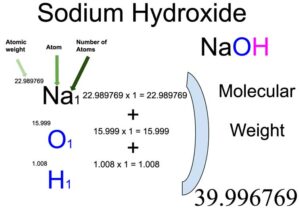
Sodium Hydroxide Naoh Molecular Weight Calculation Laboratory Notes
The values of the colligative properties are lower than expected.

. Substitute the known values to calculate the molarity. Grams of reactant used grams of product formed x 1 mol of productmolar mass of product x mole ratio of reactantproduct x molar mass of reactant. Sodium chloride carbon dioxide sulfuric acid glucose.
This means the sodium hydroxide was the limiting reactant and 4864 grams of sodium phosphate is formed. The molar mass of oxygen nitrogen and. The density of the solution is 102 g cm 3.
The molar mass of sodium hydroxide is 40 g mol 1. Download Product Safety Card. Gml Kgm3 specific gravity caustic soda density chart what is the density of sodium hydroxide.
Reduced boiling point and freezing point. You can also use this molarity calculator to find the mass concentration or molar mass. Our molar mass calculator has this for a variety of other compounds.
Download Product Safety Card. What is the minimum mass in grams of calcium hydroxide that the student must add to the water. 106462 View Pricing Availability.
Finding Molar Mass for Specific Compounds. Molar Mass of Water H2O Molar Mass of Ammonium Hydroxide AlO3H3 Molar Mass of Ammonium phosphate N3H12PO4 Molar Mass of Bismuth subsalicylate C7H5BiO4. 40 gmol Chemical Formula.
The molarity of a sodium hydroxide solution is 051 M. Sodium acetate is also used in heating pads hand warmers and hot iceSodium acetate trihydrate crystals melt at 58584 C 13641371 F dissolving in their water of crystallizationWhen they are heated past the melting point and subsequently allowed to cool the aqueous solution becomes supersaturatedThis solution is capable of cooling to room. What is the density of 51020 sodium hydroxide.
Observed molar mass is greater than the predicted value. The density of sodium hydroxide solutions. Exhaust from a chimney contains 10 mol of oxygen O 2 53 mol of nitrogen N 2 and 37 mol of carbon dioxide CO 2.
Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly. To determine the amount of excess reactant remaining the amount used is needed. A 50 ww sodium hydroxide solution means that there is 50 g of NaOH per 100 g of solution.
Sodium hydroxide is an odorless and white crystalline substance that absorbs moisture from the air at the environmental or surrounding temperature. Sodium hydroxide is a highly corrosive substance. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH.
40 gmol Chemical Formula. To learn about the structure Properties Preparation Uses Health Hazards and FAQs of Sodium hydroxide NaOH. Its a synthetic chemical.
Sodium hydroxide NaOH - Sodium hydroxide is an ionic compound. The value of i is greater than one. The molecular weight of sodium hydroxide is 40 gmol.
As mass volume molarity molar mass then mass volume molar mass molarity. The density of 50 ww Sodium hydroxide solution is 1515 gml at 25C which means that the weight of the 1 ml of Sodium hydroxide solution is 1515 g at 25C. What is 51025 NaOH density.
106498 View Pricing Availability. The observed value of molar mass is lesser than the normal value. Request More Information.
The solubility of calcium hydroxide at 25C is 012 g100 mL water. A student has been given 250 mL of water at 25C and needs to add enough calcium hydroxide to make a saturated solution. The value of the Vant Hoff factor is less than one.
Visit BYJUS for more. Worked Example 1 using the StoPGoPS approach to problem solving. Sodium hydroxide CAS 1310-73-2 pellets for analysis EMSURE - Find MSDS or SDS a COA data sheets and more information.
It is a white translucent crystalline solid and used in the manufacturing of detergents and soaps. Sodium hydroxide CAS 1310-73-2 pellets EMPLURA - Find MSDS or SDS a COA data sheets and more information. Convert the expressions above to obtain a molarity formula.
Its usually applied as. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. Molarity 5 12 3646 0114 moll 0114 M.
Molarity refers to the number of moles of the solute present in 1 liter of solution.

Molecular Weight Of Sodium Hydroxide Molecular Mass Of Naoh Molar Mass Naoh Naoh Molar Mass Youtube

Molar Mass Molecular Weight Of Naoh Sodium Hydroxide Youtube

Molar Mass Molecular Weight Of Naoh Sodium Hydroxide Youtube

Quiz Worksheet Molar Mass Study Com
0 Response to "Sodium Hydroxide Molar Mass"
Post a Comment